What is the Current State of the Ozempic Lawsuit in 2024?
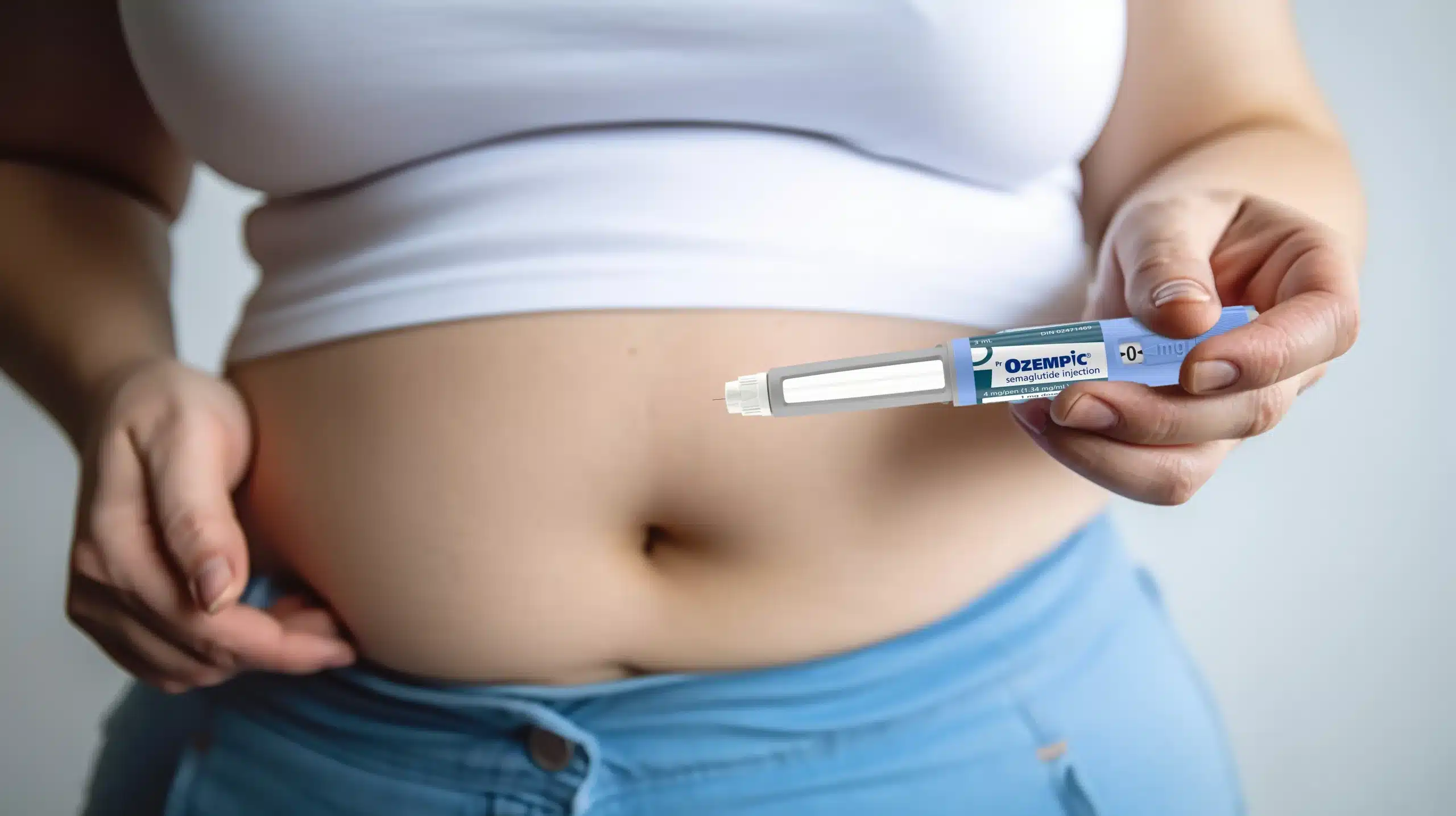
In February 2024, the legal landscape surrounding Ozempic (Semaglutide), a widely used diabetes and weight management drug, continues to evolve. RTRLAW delves into the latest developments in the ongoing litigation against Novo Nordisk, the manufacturer of Ozempic, focusing on allegations of the failure to warn patients about serious side effects, such as gallbladder disease and Gastrointestinal conditions.
Consolidation and Class Action Status Achieved
Ozempic lawsuits reached a significant milestone with the formation of a new class action lawsuit on February 2nd, 2024. The Multidistrict Litigation (MDL) Panel has directed the consolidation of federal court lawsuits relating to Ozempic under a single federal judge in Philadelphia. This move is aimed at streamlining the litigation process, given the shared factual and legal issues among the cases.
As of April 2024, Judge Gene E.K. Pratter, in the Eastern District of Pennsylvania, is presiding over 74 lawsuits concerning personal injury cases related to gastroparesis, ileus, and various forms of intestinal obstruction, all consolidated in MDL 3094.
In May 2024, Judge Gene E. K. Pratter, the presiding judge in the Ozempic lawsuit MDL, passed away unexpectedly in Philadelphia. This tragic event was anticipated to cause delays as the Judicial Panel on Multidistrict Litigation worked to reassign the case. For clients, the judge’s death might have introduced uncertainty about the progress of their lawsuits. However, lawyers involved in the litigation, reassured clients of their unwavering commitment to advancing the case. They remained diligent, ensuring that the MDL continued to make progress despite the unforeseen challenges. Prior to her passing, Judge Pratter had appointed Attorney Paul Pennock and other attorneys to the Plaintiff’s Committee to help guide the litigation.
On June 6, the Judicial Panel on Multidistrict Litigation reassigned the Ozempic MDL to Judge Karen S. Marston. Shortly thereafter, Judge Marston convened a status conference with the attorneys involved in the case. As of June 11, no official orders regarding the bellwether process set by the late Judge Pratter had been issued by Judge Marston. However, it is anticipated that Judge Marston will continue to uphold Judge Pratter’s groundwork. She is expected to collaborate with the attorneys to select cases for the bellwether test trials and oversee the coordination of discovery proceedings.
In July 2024, several significant updates emerged regarding the Ozempic lawsuits. On July 9, a new order allowed plaintiffs to directly file cases into MDL No. 3094 in the Eastern District of Pennsylvania, streamlining the process and centralizing pretrial proceedings. This order also introduced a simplified service process, with Eli Lilly and Novo Nordisk agreeing to accept email service. A status conference was scheduled for July 10 to address key issues, including discovery and causation, with plaintiffs pushing for trial readiness while defendants sought to delay litigation.
Additionally, a new study released on July 3 found a strong association between semaglutide, the active ingredient in Ozempic, and nonarteritic anterior ischemic optic neuropathy (NAION), a type of eye stroke causing sudden vision loss. This finding is significant for patients and healthcare providers when considering treatment options. Moreover, five new lawsuits were filed by July 1, bringing the total to 106, with plaintiffs alleging that the Ozempic label failed to adequately warn about its risks, leading to severe health complications.
As of August 2024, the Ozempic litigation saw several important developments. A New Hampshire resident filed a lawsuit alleging that Novo Nordisk failed to warn about the risks of Ozempic, leading to her diagnosis of gastroparesis, a condition that paralyzes stomach muscles. This lawsuit was directly filed in the ongoing MDL. Additionally, a status conference scheduled for August 8 was set to address issues such as the progress of electronic Plaintiff Fact Sheets, discovery developments, and the upcoming Science Day.
The month also saw a significant surge in the number of active cases in the Glucagon-like Peptide-1 Receptor Agonists (GLP-1 RAs) Products Liability Litigation, increasing from 111 active lawsuits in July to 346 in August, marking a 200% jump. Moreover, an Illinois man filed a new lawsuit against Novo Nordisk and Eli Lilly, claiming that their failure to warn about the risks associated with Ozempic and Mounjaro led to severe medical complications, including an ischemic bowel resection surgery. His case will be transferred to the MDL in Pennsylvania.
Warren Zeitlin
What are Some of the Legal Arguments and Company Responses to the Ozempic Lawsuit?
At the heart of these lawsuits is the accusation that Novo Nordisk failed to adequately warn users about the risks associated with Ozempic, particularly concerning Gastroparesis – a condition that can cause stomach paralysis. Despite the drug labels mentioning Gastrointestinal side effects, plaintiffs are arguing that the company downplayed the severity and risks of these issues significantly.
Geographic and Jurisdictional Challenges
The decision to consolidate the cases in Pennsylvania, close to Novo Nordisk’s headquarters, over other proposed jurisdictions like North Carolina or California, marks a strategic win for plaintiffs. This jurisdictional decision reflects the MDL panel’s consideration of the lawsuit’s logistical and legal complexities.
What Is Expected in the Ozempic Lawsuit in 2024?
Legal experts anticipate the number of lawsuits to escalate in the coming year. This increase is partly due to growing awareness among patients and healthcare professionals about the potential side effects of Ozempic and similar medications. The outcome of these legal battles could have far-reaching implications for patient safety, pharmaceutical regulations, and the drug’s future on the market.
Ozempic Recalls
In December 2023, the FDA warned consumers not to use counterfeit Ozempic (semaglutide) products found in the U.S. drug supply chain. After seizing thousands of units of the product, they are advising wholesalers, pharmacies, healthcare practitioners, and patients to check which product they have received and not distribute, use, or sell products labeled with lot number NAR0074 and serial number 430834149057.
What Are the Implications for Patients and the Pharmaceutical Industry Resulting from the Ozempic Lawsuit?
The Ozempic lawsuit underscores a critical dialogue on drug safety, corporate accountability, and the importance of transparent communication. For patients, the unfolding litigation may offer a pathway to compensation for those adversely affected. For the pharmaceutical industry, it highlights the essential balance between drug innovation and the duty to inform and protect consumers.
Contact RTRLAW for Help Today!
The state of the Ozempic lawsuit in February 2024 is a pivotal moment in pharmaceutical litigation. As the cases progress, they will not only determine compensation for affected individuals but also potentially influence how drug risks are communicated and managed in the future. Stay tuned for further developments in this significant legal battle.
If you’ve suffered severe Gastrointestinal effects from taking Ozempic, call RTRLAW now toll free at 1-833-HIRE-RTR (1-833-447-3787). Our Ozempic injury attorneys can advise you on whether you qualify for this class-action suit and will fill you in on the next steps. Contact us today for more information and free, no-obligation case review.
Revision History:
- Feb 12, 2026 at 3:32 pm by RTRLAW (displayed above)
- Feb 12, 2026 at 2:34 pm by victor

 CALL US NOW
CALL US NOW TEXT US NOW
TEXT US NOW